Linus Pauling Institute Vitamin C
Lived 1901 – 1994.
Linus Pauling was the greatest chemist of the twentieth century, and arguably ever. He was a founder of quantum chemistry, molecular biology, and molecular genetics.
To him we owe several indispensable scientific concepts including valence bond theory and electronegativity. He discovered the alpha-helix structure of proteins and discovered that sickle-cell anemia is a molecular disease.
Later in his career he became a more controversial figure. For a time the American government viewed him as a security risk. In his final years opponents labeled him a quack for championing the use of high doses of vitamin C in cancer treatments.
In an extraordinary and long life, Pauling was the sole recipient of two Nobel Prizes – an unequaled achievement – one for chemistry and another for peace.
Advertisements
Beginnings
Linus Carl Pauling was born on February 28, 1901 in Portland, Oregon, USA. He was the first of three children in the financially stretched family of Herman Henry William Pauling, a pharmaceuticals salesman, and Lucy Isabelle Darling.
From an early age Linus enjoyed mathematics and discovering how the world worked.
His father realized that Linus was unusually gifted. Unsure about how to help his son, he sent a letter to The Oregonian, a newspaper, asking for advice. Sadly, this was one of his final acts. Shortly after writing the letter he died of a perforated ulcer. It was June 1910 and Linus was nine.
Following his father's death, Linus's mother paid the bills by taking in boarders.
A Juvenile Chemist
Linus devoted himself to science against his mother's wishes – she thought a college education was a waste of time. Linus got on rather badly with his mother and sought refuge in books. Family life was bearable for him because he got on well with his two younger sisters, Pauline and Lucille.
His high school friend Lloyd Jeffress had a chemistry kit at home. Lloyd showed Linus some chemical reactions. Adding a drop of sulfuric acid to a mixture of potassium chlorate and sugar to produce water and carbon impressed Linus. He began to focus on chemistry and built a laboratory in his home's basement.
By the time he was 15, Linus had the school credits he needed to enroll at Oregon State Agricultural College in Corvallis (now Oregon State University).
Unfortunately his high school – Washington High School, Portland – refused to award him his high school diploma because he still needed two civics credits. Nevertheless Linus left school, offering to pass the exams at college, but his high school said no to this.
In 1963, his high school awarded Pauling his diploma after learning that he had won the Nobel Peace Prize: Pauling was 62 years old.
College Instructor at the Age of 18
Pauling needed to pay his own way through college. After leaving school he worked hard in a variety of jobs including milk delivery boy and shipyard laborer.
His mother helped him get a job as an apprentice machinist, which paid a good wage. She hoped this would tempt him to abandon his plans for college.
He resigned his apprenticeship when, in October 1917, age 16, he moved to Corvallis to begin a chemical engineering degree.
Pauling was a rather serious young man, endlessly worried about money. Although he was never actively anti-social, he avoided the college's social life.
He supported himself with a variety of jobs through two years of college. He then told his lecturers he needed to go back to Portland and find a job to support his sick mother. His lecturers wanted him to complete his degree – they had identified him as an extraordinarily gifted student.
The college made Pauling an assistant chemistry instructor. The 18-year-old student could now support his mother financially and continue his degree course. It was very hard work. His teaching work required about 40 contact hours a week with students.
Pauling's mother died in 1919, shortly after Pauling began work as a college instructor.
 "When I was a boy, 18 years old, I became interested in the question of just why are atoms held a certain distance apart. How far apart are they when they are bonded together and what is it that holds them at this distance? What is the chemical bond between atoms? What are the structures of molecules? They were just beginning to be determined."
"When I was a boy, 18 years old, I became interested in the question of just why are atoms held a certain distance apart. How far apart are they when they are bonded together and what is it that holds them at this distance? What is the chemical bond between atoms? What are the structures of molecules? They were just beginning to be determined."
Linus Pauling, Crusading Scientist.
1977
Physical Chemistry and Graduate School
In his final two years at college Pauling devoted himself to physical chemistry – particularly the field of chemical bonding. He wanted to understand how electrons arrange themselves around nuclei so that the overall mutual attraction allows atoms to form stable molecules.
 "Mr. Pauling possesses one of the best minds I have ever observed in a person of his age, and in many ways he is superior to his instructors."
"Mr. Pauling possesses one of the best minds I have ever observed in a person of his age, and in many ways he is superior to his instructors."
Professor Floyd Rowland, Chair of Chemical Engineering, Oregon State Agricultural College
1921
In 1922, after completing his chemical engineering degree, Pauling moved south to graduate school at the California Institute of Technology (Caltech). At Caltech he used X-ray diffraction to study the structure of crystals. He graduated in 1925 summa cum laude (the highest of honors) with a Ph.D. in physical chemistry.
European Tour – Meeting the Big Shots of Quantum Mechanics
A Guggenheim Fellowship allowed Pauling to travel to Europe for two years. There he immersed himself in quantum mechanics, using mathematics to understand the behavior of electrons in atoms.
During his tour he worked with some of the biggest names in quantum mechanics, including Niels Bohr, Erwin Schrödinger, and Arnold Sommerfeld. He spent one year in Germany, during which time he became perfectly fluent in the language. He returned to Caltech in 1927, age 26, to begin work as an assistant professor of chemistry.
Linus Pauling's Contributions to Science
Linus Pauling made highly significant breakthroughs in a remarkably wide range of scientific fields. In this he was assisted by five personal qualities:
- almost unlimited reserves of energy and enthusiasm
- an immense capacity for hard work
- extremely high intelligence
- an unquenchable thirst for new discoveries
- old fashioned pigheadedness
He got most of his new ideas accepted relatively quickly by a generally skeptical audience because of two further qualities. He was:
- a brilliant communicator
- an unusually skilled self-publicist and salesman
Chemical Bonding – a Founder of Quantum Chemistry
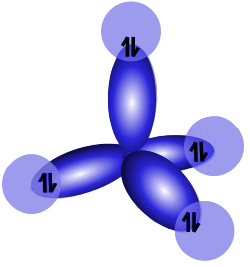
Electron orbitals in a central carbon atom hybridize to produce the familiar tetrahedral shape of methane.
Physicists had done a fantastic job of understanding quantum systems of one nucleus and one electron.
In Europe in the late 1920s Pauling began using quantum mechanics – specifically Shrödinger's wave equation – to understand more complex systems consisting of multiple nuclei and multiple electrons i.e. chemical compounds.
Pauling devised valence bond theory and gave chemistry its now everyday concepts of electronegativity, orbital hybridization, and resonance. These concepts are crucial in the teaching and understanding of how chemical compounds form and behave.
In the late 1920s Pauling published a stream of papers showing remarkable progress in understanding the structure and bonding in crystalline solids. Lawrence Bragg read these papers and was less than happy. Bragg, joint winner of the 1915 Nobel Prize in Physics for his work in X-ray diffraction and crystal structures, was in fact incensed. He believed some of Pauling's ideas were ideas he had previously published that Pauling had dressed up to look like his own.
By the early 1930s Pauling was able to show that quantum chemistry could do more than produce results that agreed with experiments. He used it to correctly predict the properties of molecules and their structures. In 1931 Caltech promoted him to full professor of chemistry.
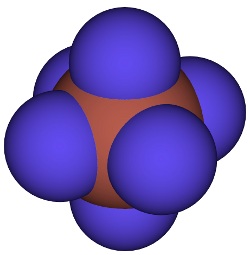
Xenon hexafluoride – predicted to exist by Pauling in 1933, first made in the 1960s. Image by CCoil.
Based on his electronegativity scale, Pauling made the unexpected prediction that the noble gas xenon could form compounds. It took three decades before experimenters were able to show Pauling's prediction was correct.
In 1939 Pauling released the twentieth century's most important chemistry book, The Nature of the Chemical Bond. With its publication, he became the twentieth century's greatest chemist.
In 1954 Pauling was the sole recipient of the Nobel Prize in Chemistry, "for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances."
Pauling had no interest in the philosophical implications of quantum theory which fascinated Niels Bohr, Albert Einstein, and many other European physicists. He took a much more practical view: it works, it helps us understand chemistry, so we use it.
 "Most scientific discoveries, made by some person say, would have been made very shortly by someone else if that person hadn't existed. And yet it seems to me that I have introduced into my work on the chemical bond a way of thinking that might not have been introduced by anyone else, at least not for quite a while. I suppose that the complex of ideas that I originated in the period around 1928 to 1933 – 1931 was probably my most important paper – has had the greatest impact on chemistry."
"Most scientific discoveries, made by some person say, would have been made very shortly by someone else if that person hadn't existed. And yet it seems to me that I have introduced into my work on the chemical bond a way of thinking that might not have been introduced by anyone else, at least not for quite a while. I suppose that the complex of ideas that I originated in the period around 1928 to 1933 – 1931 was probably my most important paper – has had the greatest impact on chemistry."
Linus Pauling, Crusading Scientist.
1977
Manhattan Project? No
Many of America's greatest scientists took part in the Manhattan Project, the Allied effort during World War 2 to build the world's first nuclear weapon. One glaring omission from the roll call was Linus Pauling.
The Project's scientific leader Robert Oppenheimer wanted Pauling, the man he saw as America's greatest chemist, to lead the project's chemistry section. Pauling refused, saying he was unwilling to disrupt his family life by moving away from Caltech. The fact that Oppenheimer had once tried to start an affair with Pauling's wife may also have something to do with the rebuff!
Despite his refusal to work for Oppenheimer, Pauling did not hide from the war effort. He worked on a number of important projects, including inventing an oxygen meter to monitor air quality in aircraft and submarines, developing armor-piercing artillery shells, formulating rocket fuel, and developing artificial blood plasma.
In 1948 President Harry Truman awarded him the Presidential Medal for Merit for his war work.
Discovery of the Protein Helix – Founder of Molecular Biology
Lying in bed one day in 1948, bored and recovering from illness, Pauling started thinking about proteins. To pass the time, he started drawing atoms and chemical bonds on paper. He then started folding the paper to match hydrogen bonds with atoms. As he did so, the shape of a helix emerged. Pauling had stumbled upon the truth about one of life's great mysteries. He later used X-ray diffraction to confirm the message from his chemical origami: proteins, the basic molecules of life, exist as helixes: the alpha-helix he discovered is a major variety of protein helix.
Protein molecules exist as helixes.
Pauling's discovery paved the way for the 1953 discovery of DNA's double-helix structure by the combined efforts of Francis Crick, Rosalind Franklin, James Watson, and Maurice Wilkins. Pauling was their only serious competitor in the race, but he incorrectly believed DNA was a triple-helix.
According to Francis Crick, Linus Pauling:
 "should be regarded as the father of molecular biology"
"should be regarded as the father of molecular biology"
Francis Crick
1986
And from James Watson:
 "There was no one like Linus in all the world. The combination of his prodigious mind and his infectious grin was unbeatable."
"There was no one like Linus in all the world. The combination of his prodigious mind and his infectious grin was unbeatable."
James Watson
1968
Sickle-Cell Anemia – Founder of Molecular Genetics
Sickle-cell anemia is a genetic disease affecting some people from parts of Africa and India or with ancestors from these regions. In order to improve resistance to malaria, red blood cells in affected people have undergone genetic change to produce a different shape – the sickle cell – as shown in the image below.
The drawback of the change is that sickle cells carry less oxygen, resulting in sickle-cell anemia.

In November 1949, Pauling and his graduate student coworkers discovered that sickle-cell anemia is caused by an abnormal protein. The electric charge on the hemoglobin of sufferers is different from that in healthy people. Sufferers have a mixture of normal red blood cells and sickle cells.
Furthermore, Pauling and his students proved that Mendelian inheritance actually works at the level of the properties of a protein molecule, not just whether the protein is present or not. In doing so, they established an enormously important new scientific discipline – molecular genetics.
Politics and the Nobel Peace Prize
A Brush with Death
Pauling's political views started as Republican and ended up left wing. One of the key players in this shift was Thomas Addis, an eminent doctor specializing in nephrology.
In 1940 Pauling fell dangerously ill with glomerulo nephritis, a kidney disease considered fatal in the 1940s. Addis treated Pauling for a number of years, a large part of which involved regulating his patient's diet and nutrition. Pauling, always looking for interesting phenomena, noted the strong links between nutrition, disease, and health.
Pauling survived. He believed he owed his life to Addis's devoted work as a physician. Addis, however, was a communist. The government had declared him a security risk, making his life difficult at times. This distressed Pauling.
Sympathy for Japanese in America
Pauling was also distressed by other events he saw as unjust in the 1940s, such as the World War 2 internment of Americans with Japanese ancestry. During the war he and his wife hired a gardener whose parents were Japanese. Someone resented this enough to paint anti-Japanese slogans on the Paulings' garage and threaten them anonymously over the phone.
Although very fearful, the couple did not back down: their gardener left only when he was summoned to join the US Army.
The Nobel Peace Prize

Pauling believed all warfare to be irrational, particularly nuclear warfare.
In the 1950s, during the cold war, Pauling was increasingly public in his support for anti-government causes. The result of this was that he ended up facing travel restrictions.
In 1957 he was instrumental in gathering a petition to ban nuclear weapons testing: it was signed by over 11,000 scientists from 50 countries. Later the United States, the United Kingdom, and the Soviet Union agreed to a partial nuclear test ban, mainly as a result of the petition.
Pauling received the 1962 Nobel Peace Prize. The Nobel committee noted that he had:
"…campaigned ceaselessly, not only against nuclear weapons tests, not only against the spread of these armaments, not only against their very use, but against all warfare as a means of solving international conflicts."
Pauling was attacked mercilessly in a number of publications for his views. The New York Times was less heated, commenting in an editorial:
"Professor Pauling has been a persistent and effective agitator in his fight for international peace and against the atmospheric testing of nuclear weapons. He has not always been wise in his choice of tactics and he has sometimes been reckless."
Pauling was often accused of being a communist or Soviet sympathizer, but he was actually too much of a free spirit to ever support any form of totalitarian government. It's probably fair to say, however, that he would have chosen to sacrifice western freedoms if a nuclear war were the price of defending them.
Although Pauling never mixed politics with his classes at Caltech, his political statements on issues such as 'ban the bomb' eventually caused so much strain with Caltech's administrators that in 1964, age 63, Pauling left Caltech to work at the Center for Study of Democratic Institutions in Santa Barbara.
Back to Chemistry and Curing Cancer with Vitamin C
From 1967 to 1969 Pauling was a professor of chemistry at the University of California, San Diego.
Between 1969 and 1974 he was a professor of chemistry at Stanford University.
Since his brush with death in the 1940s, Pauling had become increasingly interested in the relationship between nutrition and health. In 1970 he published a bestseller, Vitamin C and the Common Cold.
In 1973 he founded the Linus Pauling Institute of Science and Medicine at Menlo Park, California. The main focus of his work was researching the health benefits of large doses of vitamin C, particularly in treating atherosclerosis, heart disease, and cancer.
He organized treatments involving very large doses of vitamin C for cancer patients. He and his team got positive results.
The results were rebutted by researchers from the Mayo Clinic, who said they could not replicate them.
The Mayo Clinic's rebuttal was then rebutted by Pauling, who said the Mayo Clinic had not followed the same vitamin C dosing regime he had used, and they had ended vitamin C dosing after less than three months, rather than using it as a long term treatment.
Pauling's vitamin C claims remain controversial.
The Linus Pauling Institute still exists, researching the links between nutrition and health. It is now based at Pauling's Alma Mater, Oregon State University.
Some Personal Details and the End
Pauling met his future wife Ava Helen Miller in his final year of college in Oregon. She was a student in one of the chemistry classes he taught.
They married in 1923 when he was 22 years old. Three of their four children pursued scientific careers: Linus Jr. became a psychiatrist; Peter a crystallographer; Edward a biologist. Their daughter Linda married geologist Barclay Kamb. Linus and Ava's children gave them 15 grandchildren.
Ava encouraged Pauling's forays into politics. He said she should have shared his Nobel Peace Prize.
Linus Pauling died of prostate cancer on August 19, 1994 in Big Sur, California. He was 93 years old. His ashes and those of his wife were buried in Oswego Pioneer Cemetery, Lake Oswego, Oregon in 2005.
Advertisements
Author of this page: The Doc
Images digitally enhanced and colorized by this website. © All rights reserved.
Cite this Page
Please use the following MLA compliant citation:
"Linus Pauling." Famous Scientists. famousscientists.org. 10 Jun. 2016. Web. <www.famousscientists.org/linus-pauling/>.
Published by FamousScientists.org
Further Reading
Anthony Serafini
Linus Pauling A Man and His Science
Paragon House, 1989
John Horgan
Stubbornly Ahead of his Time
Scientific American 266, 36 – 40, 1993
Thomas Hager
Force of Nature: The Life of Linus Pauling
Simon & Schuster, 1995
Bruno J. Strasser
Linus Pauling's "Molecular Diseases": Between History and Memory
American Journal of Medical Genetics (Semin. Med. Genet.) 115:83–93, 2002
Linus Pauling Institute Vitamin C
Source: https://www.famousscientists.org/linus-pauling/

Tidak ada komentar: